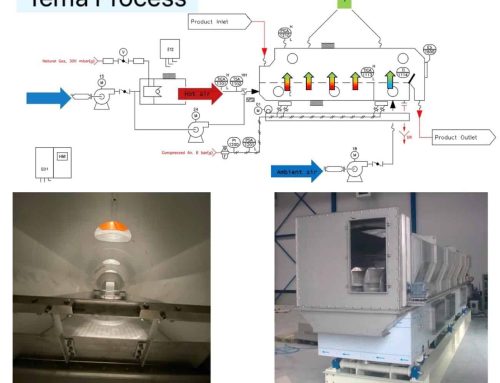
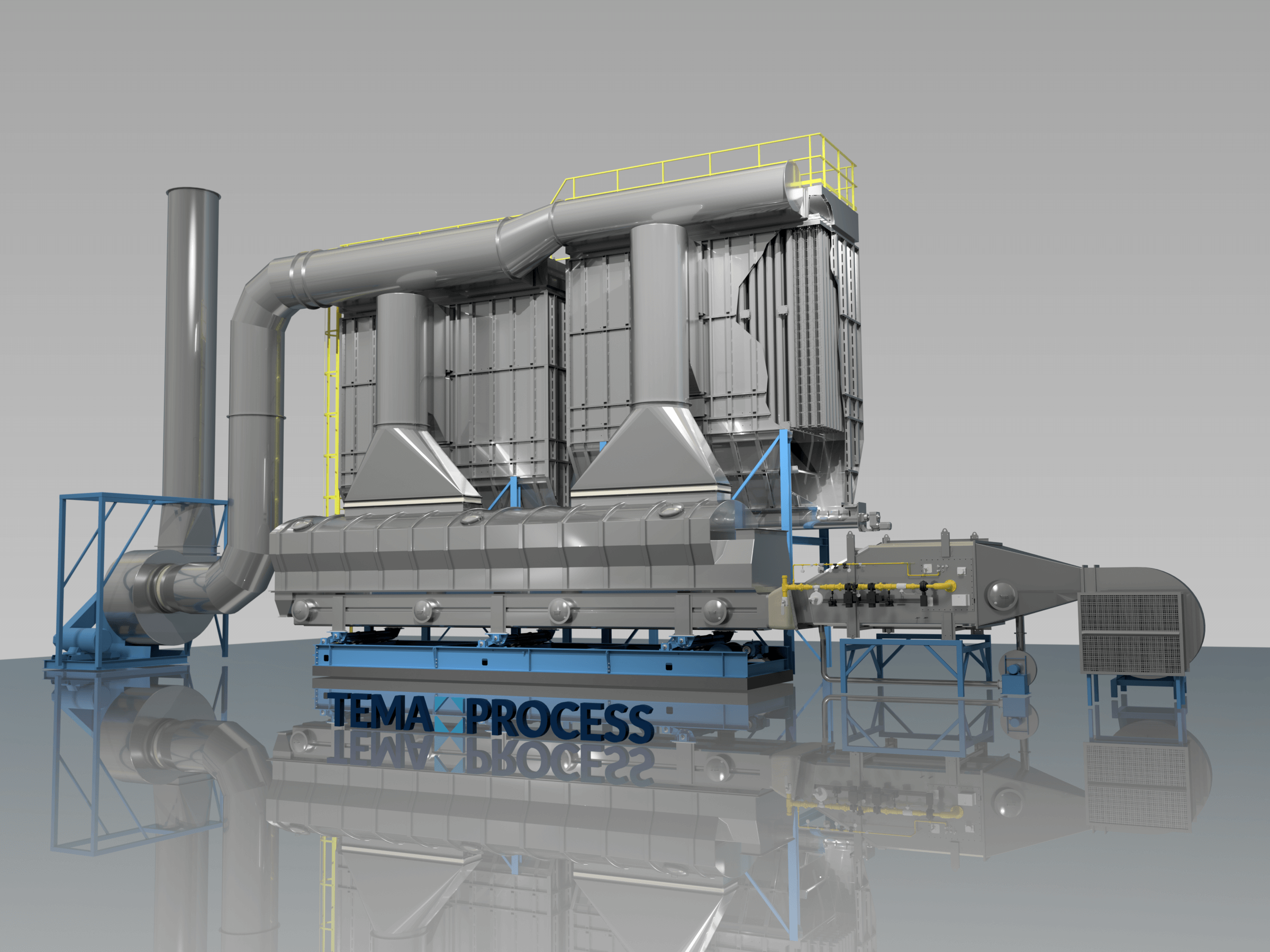
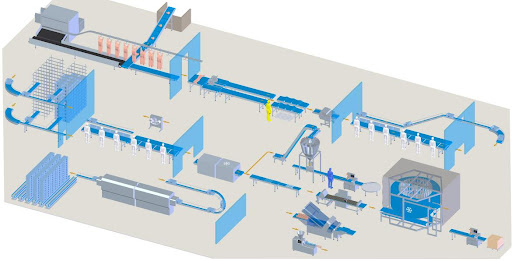
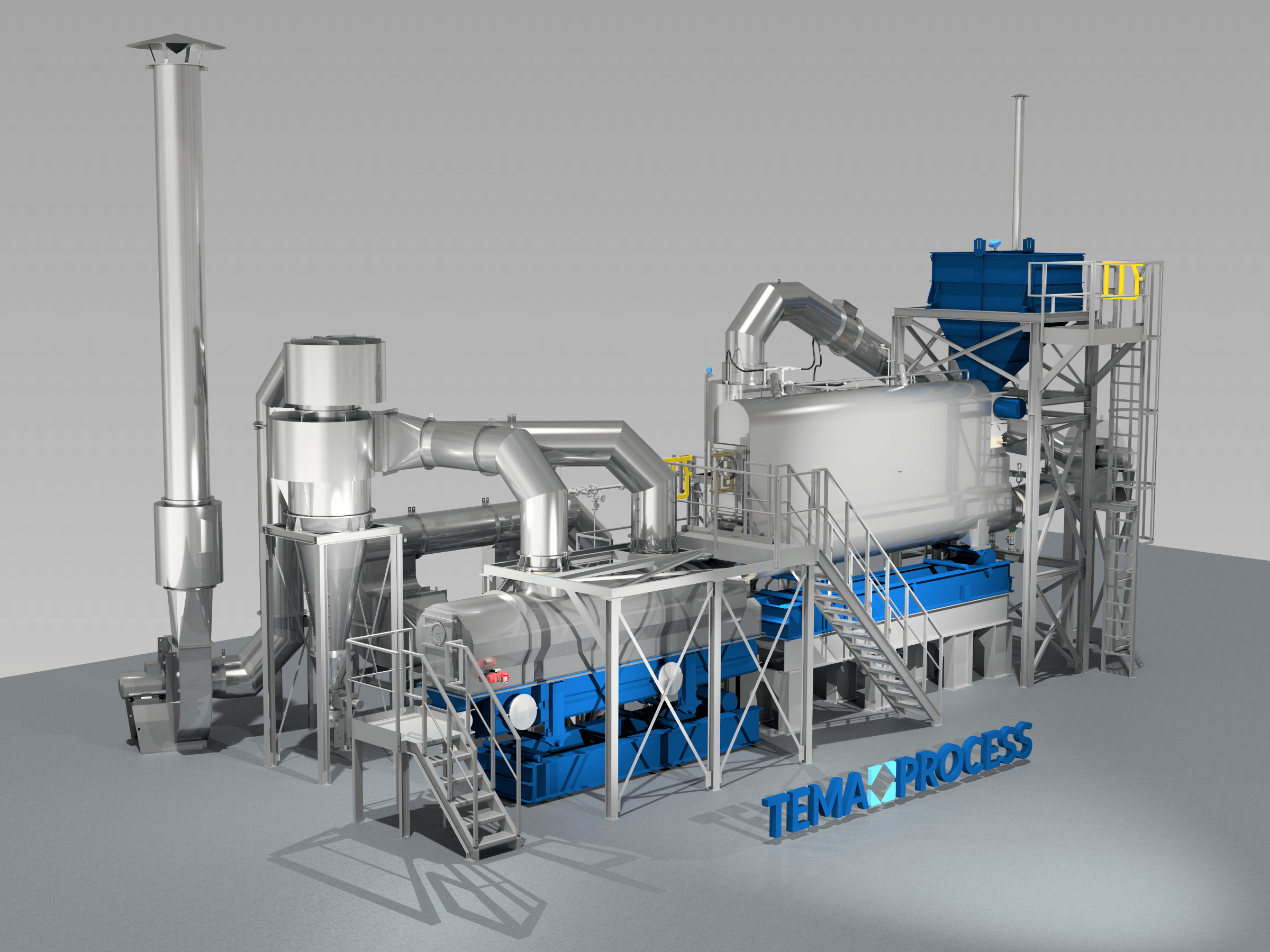
Drying : Ammonium sulfate [(NH4)2 SO4] or AMSUL was one of the first and most widely used nitrogen (N) fertilizers for crop production. It’s now less common but especially valuable where both N and sulfur (S) are required. Its high solubility provides versatility for a number of agricultural applications. Production: Ammonium sulfate (sometimes abbreviated as AS or AMS) has been produced for more than 150 years. Initially, it was made from ammonia released during manufacturing coal gas (used to illuminate cities) or from coal coke used to produce steel. Today, manufacturers make it by reacting sulfuric acid with heated ammonia. To get the crystal size best suited for the application, they control the reaction conditions by screening and drying the particles until achieving the desired size. Some materials are coated with a conditioner to reduce dust and caking.





![Drying Ammonium sulfate [(NH4)2 SO4] or AMSUL](https://temaprocess.com/wp-content/uploads/2019/02/DryingAmmonium-sulfate-NH42-SO4-or-AMSUL.jpg)
![Shipping dryers [video]](https://temaprocess.com/wp-content/uploads/2020/08/shipping-dryers-500x383.png)
![Increasing production capacity [video]](https://temaprocess.com/wp-content/uploads/2020/06/Increasing-production-capacity-video-500x383.png)
![Fluid bed dryers for spray drying [video]](https://temaprocess.com/wp-content/uploads/2020/06/Fluid-bed-dryers-for-spray-drying-500x383.png)